Cybin Initiates PARADIGM: A Multinational Pivotal Phase 3 Program Evaluating CYB003 for the Adjunctive Treatment of Major Depressive Disorder and Reports Second Quarter Financial Results
- PARADIGM ™ program comprises two 12-week randomized, placebo-controlled studies (APPROACH and EMBRACE) and a long-term extension study (EXTEND) -
- APPROACH ™: A Phase III, Placebo-Controlled, Randomized, Double-Blind Trial of Oral Doses of CYB003 to Assess Combined Safety and Efficacy in Humans with Major Depressive Disorder has been initiated and will enroll 220 patients at 36 clinical sites across the U.S. and Europe; topline results expected in 2026 -
- EMBRACE ™: An Efficacy and Safety, Phase III, Multi-center, Double-Blind, Randomized Controlled Study Comparing 2 Active and 1 Inactive Oral Doses of CYB003 in Eligible Participants with Major Depressive Disorder is expected to begin in 1H25 -
- EXTEND: a Phase III Open Label Extension Study with Optional Additional Doses of CYB003 to Assess the Safety and Long-term Efficacy in Participants With Major Depressive Disorder is expected to begin 12 weeks after commencement of APPROACH and EMBRACE, respectively -
-12-month efficacy data from Phase 2 CYB003 MDD study expected in Q4 2024 -
- CYB003 in development for the treatment of MDD has a total addressable market of >300 million people worldwide1 and 21 million in the U.S. 2 -
- Cash totaled C$154.3 million as of September 30, 2024 –
This news release constitutes a “designated news release” for the purposes of Cybin’s prospectus supplements each dated August 23, 2023, to its short form base shelf prospectus dated August 17, 2023, as amended December 22, 2023 and April 8, 2024.
TORONTO--(BUSINESS WIRE)-- Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced the initiation of PARADIGMTM, its Phase 3 pivotal program evaluating the efficacy and safety of CYB003 for the adjunctive treatment of Major Depressive Disorder (“MDD”). The program name, PARADIGM, represents the Company's belief that CYB003 could have the potential for a paradigm shift in the treatment of depression. The Company also today reported unaudited financial results for its second quarter ended September 30, 2024.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241113365253/en/
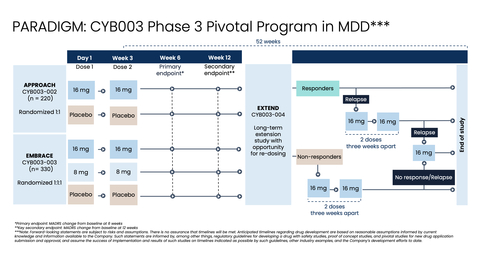
Phase 3 PARADIGM Pivotal Program Design (Graphic: Business Wire)
“Just three years after filing an Investigational New Drug application for CYB003, the initiation of our Phase 3 program is a truly significant and gratifying milestone,” said Doug Drysdale, Chief Executive Officer of Cybin. “Following a highly collaborative and thorough design and review process with the U.S. Food and Drug Administration, we believe that PARADIGM incorporates appropriate protocols that proactively address some of the challenges encountered by peers developing molecules with similar mechanisms of action by (i) recruiting from the larger MDD population; (ii) administering CYB003 as an adjunctive treatment and not requiring patients to titrate off their existing antidepressants; and (iii) utilizing a 12-week blinded period to maximize the number of patients that remain in the study through the blinded stage. Our innovative approach represents a potential ‘paradigm’ shift, moving away from the daily treatment of depression symptoms and toward an intermittent, more durable treatment like CYB003 that could potentially change the course of the disease. Our clinical team has accomplished an extraordinary amount in a short time, and we are eager to continue investigating CYB003’s potential to provide long-lasting relief from depressive symptoms and disrupt the standard of care in MDD,” concluded Drysdale.
About the Phase 3 PARADIGM Pivotal Program
The Company’s Phase 3 program comprises three pivotal efficacy studies:
Pivotal study 1 (APPROACH):
- Participants (n=220) will be randomized 1:1 to receive either 16 mg of CYB003 (n=110) or inactive placebo (n=110). Each study arm will evaluate a two-dose regimen, with doses administered three weeks apart. The study will enroll patients suffering from moderate to severe MDD (MADRS≥24) who are on a stable dose of antidepressant medication but are responding inadequately.
- The primary endpoint will be change in depressive symptoms as measured by change in MADRS from baseline at six weeks after the first dose.
APPROACH will enroll participants at 36 clinical sites across the U.S. and Europe.
Pivotal study 2 (EMBRACE):
- Participants (n=330) will be randomized 1:1:1 to receive 16 mg of CYB003 (n=110), 8 mg of CYB003 (n=110), or inactive placebo (n=110). Each arm will evaluate a two-dose regimen, with doses administered three weeks apart. The study will enroll patients suffering from moderate to severe MDD (MADRS≥24) who are on a stable dose of antidepressant medication but are responding inadequately.
- The primary endpoint will be change in depressive symptoms as measured by change in MADRS from baseline at six weeks after the first dose.
EMBRACE is expected to enroll at 48 clinical sites, with minimal site overlap with the APPROACH study.
Pivotal study 3 (EXTEND):
- Participants from APPROACH and EMBRACE will roll over into EXTEND (up to n=550) after the completion of the 12-week, double-blind, placebo-controlled treatment periods. During EXTEND, all participants who did not respond to treatment in the APPROACH and EMBRACE studies or who relapse during the EXTEND study will be eligible to receive an additional two doses of CYB003 (16 mg) administered three weeks apart. Participants who do not respond to these two doses or relapse again will be eligible to receive an additional single 16 mg dose of CYB003.
Across all three studies, raters will be remote, independent, and blinded with no information on the dose received or the participant’s dosing experience. Effects during the dosing session will be firewalled to ensure that the study team stays blinded.
“Our unique Phase 3 pivotal program design has been informed by the impressive Phase 2 (four-month) data showing rapid, robust improvements in symptoms of depression with a single dose of CYB003, and durable effects four months after two doses with a 75% remission rate in the 16mg dose group. For our pivotal program, we have preserved the two-dose regimen used in our Phase 2 study, given the strong durability results seen to date,” said Amir Inamdar, Chief Medical Officer of Cybin. “The need for improved treatments for mental health disorders has never been greater. We believe that our Phase 3 program can build on the positive results demonstrated in Phase 2 to-date and could potentially lead to the approval and commercialization of a novel treatment modality whose effects are consistent and durable for patients with MDD.”
Positive Phase 2 Four-Month Efficacy Data for CYB003 in MDD
-
Robust and sustained improvements in symptoms of depression with two doses of 12 mg or 16 mg of CYB003:
- Mean reduction from baseline in the MADRS total score was approximately 22 points from baseline in both dosing cohorts.
- Approximately 75% of the patients were responders (>/= 50% improvement in MADRS scores) following two doses of 16mg.
-
75% of patients on 16 mg were in remission from depression following 2 doses (MADRS score
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.