Sanofi SNY announced that the European Commission has approved Teizeild (teplizumab) to delay the onset of stage 3 type 1 diabetes (T1D) in patients aged eight years and older with stage 2 disease. The approval was expected as the Committee for Medicinal Products for Human Use of the European Medicines Agency had issued a positive opinion in November recommending the approval of Teizeild.
With this approval, Teizeild becomes the first T1D disease-modifying drug authorized in the European Union.
The drug is already approved in the United States and several other countries for delaying the onset of stage 3 T1D in adults and children aged eight years and older with stage 2 T1D. Sanofi markets the drug under the brand name of “Tzield” outside the European Union.
In the past year, SNY’s shares have declined 1.7% against the industry’s 21.9% rise.
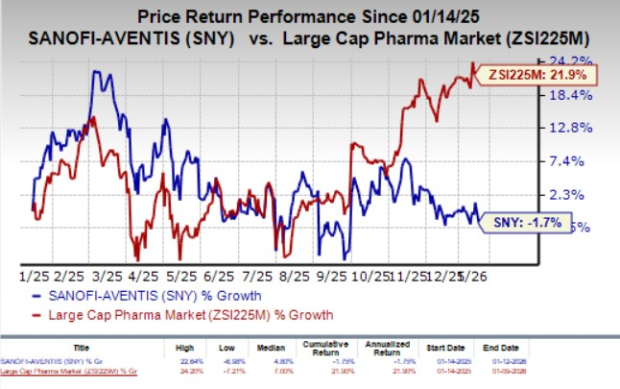
Image Source: Zacks Investment Research
SNY’s Teizeild Approval Based on TN-10 Phase II Study
The European Commission’s approval is supported by positive data from the TN-10 phase II study, which included 76 participants aged eight to 45 years with stage 2 T1D. Participants were randomly given either Teizeild or a placebo for 14 days.
The study met its primary endpoint by demonstrating significant delays in progression from stage 2 T1D to stage 3 T1D. The median time to progression to stage 3 diabetes was 48.4 months in the Teizeild arm versus 24.4 months in the placebo arm. Moreover, 57% of patients treated with Teizeild remained in stage 2 compared with 28% in the placebo group, with fewer adverse events reported.
Teizeild was added to Sanofi’s portfolio in 2023, following its $2.9 billion acquisition of Provention Bio. The drug was originally developed by MacroGenics MGNX. Provention Bio acquired this drug from MacroGenics under an asset purchase agreement signed in 2018.
Earlier this month, the FDA granted priority review to a regulatory filing seeking approval to expand Tzield’s use in the United States for individuals aged one year and older with stage 2 disease. The final decision is expected by April 29, 2026.
Tzield recorded global product sales of €47 million in the first nine months of 2025, up 33.3% at constant exchange rates, with €44 million coming from the United States.
Sanofi’s Zacks Rank & Stocks to Consider
SNY currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Amicus Therapeutics FOLD and Indivior INDV, currently sporting a Zacks Rank #1 (Strong Buy) each. You can see the complete list of today’s Zacks #1 Rank stocks here.
Over the past 60 days, estimates for Amicus Therapeutics’ 2026 earnings per share have declined from 67 cents to 65 cents. Shares of FOLD have increased 55% over the past year.
Amicus Therapeutics’ earnings beat estimates in one quarter and missed in the remaining three trailing quarters, with the negative average surprise being 20.21%.
Over the past 60 days, estimates for Indivior’s 2026 earnings per share have risen to $2.85 from $2.60. Indivior stock has rallied 208.3% over the past year.
Indivior’s earnings beat estimates in three of the trailing four quarters and were in line in the remaining quarter, with the average surprise being 68%.
Zacks' Research Chief Names "Stock Most Likely to Double"
Our team of experts has just released the 5 stocks with the greatest probability of gaining +100% or more in the coming months. Of those 5, Director of Research Sheraz Mian highlights the one stock set to climb highest.
This top pick is a little-known satellite-based communications firm. Space is projected to become a trillion dollar industry, and this company's customer base is growing fast. Analysts have forecasted a major revenue breakout in 2025. Of course, all our elite picks aren't winners but this one could far surpass earlier Zacks' Stocks Set to Double like Hims & Hers Health, which shot up +209%.
Free: See Our Top Stock And 4 Runners UpSanofi (SNY) : Free Stock Analysis Report
Amicus Therapeutics, Inc. (FOLD) : Free Stock Analysis Report
MacroGenics, Inc. (MGNX) : Free Stock Analysis Report
Indivior PLC (INDV) : Free Stock Analysis Report
This article originally published on Zacks Investment Research (zacks.com).
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.


