Shares of Altimmune ALT fell 27.04% during market hours on Sep 14, even after the company announced positive top-line results from its 12-week phase I study. The study evaluated pemvidutide in non-alcoholic fatty liver disease (NAFLD) patients.
Formerly known as ALT-801, pemvidutide is an investigational, peptide-based GLP-1/glucagon dual receptor agonist. It is currently being developed to treat obesity and non-alcoholic steatohepatitis (“NASH”).
The randomized, double-blind placebo-controlled phase I study is evaluating three dose cohorts of pemvidutide, namely 1.2mg, 1.8mg and 2.4 mg, against placebo in 94 NAFLD patients.
The study achieved its primary endpoint of relative and absolute reductions in liver fat in all the three dose cohorts. In the 1.8 mg dose group, the candidate achieved 68.5% mean reduction in liver fat content, out of which 94.4% of the subjects achieved a 30% reduction in liver fat, 72.2% of the patients achieved a 50% reduction, while 55.6% patients achieved normalization of liver fat.
Moreover, mean alanine aminotransferase (ALT) also lowered in all the study subjects. Liver enzyme levels declined more than 17 IU/L at all dose levels in patients with baseline serum ALT above 30 IU/L and declined 27 IU/L in the 2.4 mg dose cohort.
The study also met its secondary endpoint in all three dose cohorts of pemvidutide. The secondary endpoint was the percentage of weight loss from the baseline in the subjects for a 12-week treatment.
Subjects without diabetes witnessed a mean weight loss of 4.9% at the 1.8mg and 2.4 mg doses against a 4.7% weight loss in the placebo-controlled group. While subjects with diabetes recorded a decline of 4.4% in weight at the 1.8mg and 2.4 mg dose cohorts, against the 3.9% weight loss in the placebo-controlled group.
The company reported that pemvidutide was generally well tolerated in the subjects. Two subjects treated with pemvidutide dropped out of the study due to side effects.
Altimmunbe expects that the positive results from the study, which reduced liver fat and serum ALT together in just 12 weeks in NAFLD patients, could be a promising drug therapy for NASH and obesity.
The company also anticipates reporting data from the 24-week NAFLD study and interim data from its 24-week MOMENTUM obesity study in the near term.
However, Altimmune’s data from the early-stage clinical study for NASH did not impress the investors, thereby causing a fall in Altimmune’s share prices.
Nonetheless, shares of Altimmune have returned 62.3% in the year-to-date period, against the industry’s decline of 22.6 %
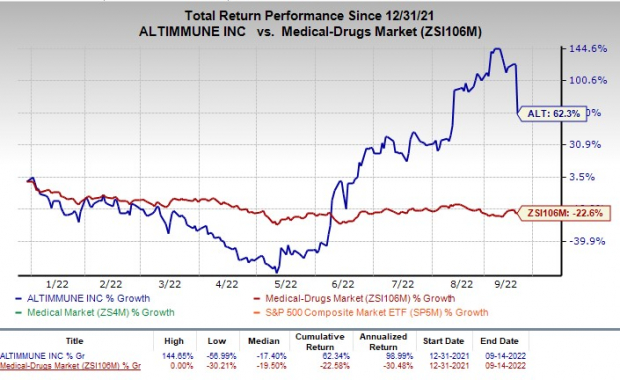
Image Source: Zacks Investment Research
With no approved therapies yet, the NASH market holds tremendous potential, attracting several players in the industry.
Intercept Pharmaceuticals ICPT is also evaluating its obeticholic acid for NASH. Though the company received a complete response letter (CRL) from the FDA regarding its new drug application (NDA) for obeticholic acid for treating NASH, it is currently resubmitting its NDA by the end of 2022 after having a pre-submission meeting with the FDA.
Intercept’s NDA resubmission for obeticholic acid for treating NASH is based on the positive top-line data from a new interim analysis of its ongoing pivotal phase III REGENERATE study, evaluating obeticholic acid in NASH.
Only yesterday Akero Therapeutics AKRO reported top-line data from its mid-stage HARMONY study evaluating its lead-candidate efruxifermin (EFX) in patients with NASH, fibrosis stage 2 or 3 (F2-F3). Akero’s lead product candidate met both its primary and secondary endpoints in the multicenter, placebo-controlled phased II study, evaluating EFX in two dose cohorts, namely 28mg and 50 mg, in 128 patients for six months.
Altimmune, Inc. Price

Altimmune, Inc. price | Altimmune, Inc. Quote
Zacks Rank and A Key Pick
Altimmune currently has a Zacks Rank #3 (Hold).
A better-ranked stock in the same sector is Acer Therapeutics ACER, carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Acer Therapeutics’ loss per share estimates for 2022 have improved from $3.47 to $2.47 in the past 30 days. The same for 2023 has improved from $1.49 to $1.07 in the same time frame.
Earnings of Acer missed estimates in two of the trailing four quarters and beat the same on the remaining two occasions. The average negative earnings surprise for ACER is 106.16%.
Free Report Reveals How You Could Profit from the Growing Electric Vehicle Industry
Globally, electric car sales continue their remarkable growth even after breaking records in 2021. High gas prices have fueled his demand, but so has evolving EV comfort, features and technology. So, the fervor for EVs will be around long after gas prices normalize. Not only are manufacturers seeing record-high profits, but producers of EV-related technology are raking in the dough as well. Do you know how to cash in? If not, we have the perfect report for you – and it’s FREE! Today, don't miss your chance to download Zacks' top 5 stocks for the electric vehicle revolution at no cost and with no obligation.
>>Send me my free report on the top 5 EV stocksWant the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Altimmune, Inc. (ALT): Free Stock Analysis Report
Intercept Pharmaceuticals, Inc. (ICPT): Free Stock Analysis Report
Acer Therapeutics Inc. (ACER): Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.


